Principal Consultant
Dr Chris Alderman B Pharm PhD FHSP BCPP BPCG Dip Proj Man
Australian Medication Safety Services
Expert Advice on Medication Matters
The safety and wellbeing of patients are our foremost priority. Any action or recommendation that could compromise consumer protection will never be considered as an option
The safety and wellbeing of practitioners/staff involved in medication use are also paramount considerations, the organisation will never create a serious adverse impact on the safety, health or wellbeing of these other stakeholders
Providing good value for money is important – clients should feel satisfied that they have received good work for a fair price, delivered on time
The organisation is guided by the key principles of acting ethically, honestly and with integrity, basing all recommendations and actions on these values;
Information about safe and effective use of medications evolves rapidly – we maintain timely access to high quality and authoritative sources in medical, nursing and pharmacy literature and frequently update resources and position statements that are made availabel to clients using the group’s services
News and resources section
Medication outperforms non-medication treatment for opioid use disorders
While methadone and buprenorphine treatment for opioid use is widely used and accepted, the efficacy of non-medication treatments in reducing opioid use and improving treatment retention is unclear. A retrospective cohort study in the United States has compared...
Paediatric antibiotic prescribing is amenable to interventions to reduce rates of usage
The efficacy of a program aimed to reduce inappropriate outpatient antibiotic prescribing for paediatric acute respiratory tract infections (ARTIs) has been evaluated in a recently published stepped-wedge clinical trial. 72,723 ARTI visits by 29,762 patients were...
Opioids after acute musculoskeletal injuries in adults – what predicts ongoing use?
A systematic review has examined factors associated with persistent opioid use after prescribing for acute musculoskeletal injury. Studies involving 263 393 participants were included, and the overall prevalence of prolonged opioid use after musculoskeletal injury for...
Assessment of medication safety
A range of factors influence the way we interpret medication safety data:
Clinical trials
By necessity, the trials focus exclusively on the efficacy & safety of the drug when used for a specific clinical indication, and do not necessarily address other areas of potential clinical use – this means that as years go by, some medications start to be widely used for non-approved indications that are not directly related to the parameters of the original research. Clinical trials tend to focus upon a relatively small cohort to people that are not necessarily representative of wider society – true patterns of adverse effects or drug interactions are often not apparent in the context of a clinical trial, no matter how large – the world’s community of clinicians relies upon post-marketing surveillance to uncover these effects, where patterns emerge after the use of medications of a period of years.

Medical Factors
It is well known that the likelihood of adverse drug reactions and serious drug interactions increases in a non-linear fashion, so as the number of medications used increases, the risk of medication-related problems rises dramatically. The these reasons it is important to work towards deprescribing (www.nps.org.au/australian-prescriber/articles/deprescribing) and make efforts to avoid polypharmacy (www.nps.org.au/australian-prescriber/articles/the-dilemma-of-polypharmacy)
There are many other specific factors that impact upon medication safety for an individual patient – these can be assessed with a systemic medication review.
Institutional Factors
In addition to ensuring the safe distribution of medications it is also vital appropriate oversight of medication use must be maintained. Many of the functions required for this are provided through the delivery of clinical pharmacy servcies (www.shpa.org.au/resources/standards-of-practice-for-clinical-pharmacy-services) . These services are a vital component for ensuring safe and effective medication use. In addition, the operation of multidisciplinary governance committess is also vital – for example Drug & Therapeutics Advisory Committees (DTACs) and Medication Advisory Committees (MACs) in hospitals and Aged Care respectively.
All medicines have the potential to do harm
Reducing potential for medication-related problems
Australian Medication Safety Services
Safe and effective medication requires careful and systemic attention
All medicines have the potential to do harm
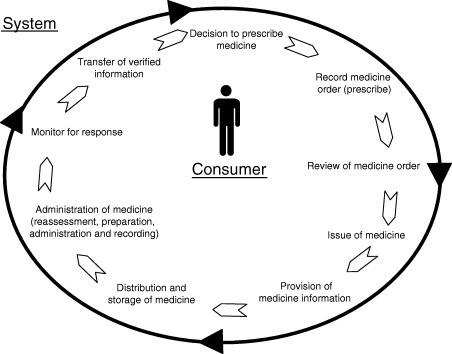
Some of the side effects associated with medications are relatively predictable, and information about these problems should be considered when making decisions about which medicines to use for an individual. At times, however, the adverse impacts associated with medications are not necessarily predictable based on simple factors or previous experience. Given that medications are very widely used, have the potential to do great good as well as significant harm, and are associated with significant expense it is important that steps are taken to maximise the likelihood a benefit and minimise the risks associated with these products. Many factors need to be taken into account when considering the issues that influence drug safety. In an individual sense these include modifiable factors that need to be considered when formulating a treatment plan for individual. These are based on these so-called “medication-related problems” (refer www.ncbi.nlm.nih.gov/pubmed/2275235) Factors that need to be considered by doctors pharmacists, and other people such as nurses who are involved in providing safe and effective care for patient are broadly based on the principles of Quality Use of Medicines (refer below)
Contact Australian Medication Safety Services
PO Box 179, Glen Osmond, South Australia
Email admin@medsafetyaustralia.com.au
